Numerous chemical formulas and symbols become easy to remember and usable in everyday environment when the essence of their meaning and purpose is understood through practical, interactive teaching.
Therefore, for the purpose of understanding of the concept of substance in chemistry, Savremena International School’s students learned about the unit for quantity in an interesting chemistry lesson, using different sorts of bulk food.
 Unusual assignment for understanding the lesson on mole
Unusual assignment for understanding the lesson on mole
The concept of the mole as such used to be quite a chore for young chemists just becoming acquainted with it. However, the students of Savremena International School had no trouble understanding the definition and importance of one of the seven basic units of the SI system through participation in a seemingly unusual assignment for a chemistry class.
Each student received a bag with a certain number of candies, macaroni, corn kernels, beans, peppers or peanuts, representing the number of particles. The task was to count the contents found inside the bag; when they announced the result, they realised that they had 40 “particles” each.
Same number of particles, different weight
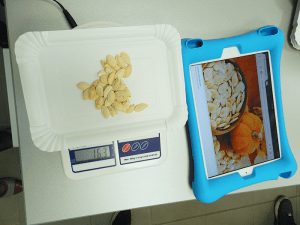 The next step was to measure the bags. Although they all had the same number of “particles”, when their contents were placed on a scale, it turned each had its own, different weight; 40 candies from one bag weighed more than 40 corn kernels from another. The difference in weight represented the difference in the number of particles in each of these substances.
The next step was to measure the bags. Although they all had the same number of “particles”, when their contents were placed on a scale, it turned each had its own, different weight; 40 candies from one bag weighed more than 40 corn kernels from another. The difference in weight represented the difference in the number of particles in each of these substances.
In this simple way, the students understood the essence of the lesson on mole, the relationship between mole and number of particles, and the reasoning behind introducing mole as the universal unit of quantity.
Solid foundation for new knowledge in chemistry
Through encountering the subject matter of stoichiometry through an interesting experiment, the students mastered the basics of this field, whose purpose is quantitative measurement of the substances involved in chemical reactions in an easy and thorough way. Having adopted the lesson on mole with full understanding, the students are now ready to step further into the world of stoichiometry and start doing some serious calculations in chemistry.
More pictures in our gallery.